Grow Bigger: The Power of Rest in Your Training.
Author:
Julio Valero
Published on:
3/27/2025

Repeated and interrupted resistance exercise induces desensitization and resensitization of mTOR-related signaling in human skeletal muscle fibers.
A recent study reveals new details about the molecular changes that muscle cells experience when training is discontinued. Although the data are preliminary, they are consistent with previous observations from research in humans.
Overview
Analyzing muscle biopsies from the vastus lateralis muscle, the researchers investigated how long it took for anabolic signaling proteins to deactivate after five weeks of training. They also assessed whether ten days without training allowed these proteins to recover their activity.
They observed that after seven training sessions, anabolic signaling proteins related to mTOR decreased, but recovered after ten days without training. Despite this reduction in signaling proteins, myofiber diameter remained unchanged during the detraining period.
How does this apply to you? Reducing intensity or taking scheduled breaks from training could offer several advantages. However, this strategy is relatively new, and more research is needed to establish clear guidelines on how to implement mesocycles and deloads that promote sustained muscle growth.
What's the problem?
Do you remember your early days with weight training? It was incredible to see how each week you could increase the weight on the bar and still do the same or even more reps. Within a few months, your physique changed noticeably. After half a year, although the weekly progress was no longer as evident, you continued to advance month after month, adding weight and seeing changes. Don't you remember the excitement and desire that training generated? With years of consistency, progress slows, personal records are less frequent, and physical changes seem to stop. This decrease in progress, despite training regularly, is due to a lower anabolic response to exercise. But imagine being able to reactivate your body's sensitivity to the anabolic effects of weight training? That would be fantastic, wouldn't it?
Resistance training triggers an anabolic response with multiple physiological consequences. However, this review focuses primarily on how resistance training influences muscle protein synthesis (MPS). To understand this effect, it is crucial to examine the sequence of signals that culminate in an increase in MPS. Simply put, "translation initiation" is the step that determines the rate of MPS. In simple terms, translation is the process by which cells use the genetic information contained in messenger RNA (mRNA) to build proteins. Ribosomes are the "machines" that read mRNA and assemble amino acids in the correct sequence to form a protein. The initiation of this process, where the ribosome binds to the mRNA, is a key step that controls the rate of protein production. This initiation step is driven by a signaling pathway called mTOR, which facilitates the binding of certain proteins (eIF4G and eIF4E) and activates others (p70S6K and p70S6). The activation of the latter, in turn, increases the production of ribosomal components, accelerating protein synthesis.
Although training induces greater activation of muscle protein synthesis (MPS) and the mTOR pathway in trained individuals, this anabolic response is shorter-lived than in beginners. This reduction in the window of muscle growth is thought to explain why more experienced individuals experience less hypertrophy with training. The study presented here sought to determine when this anabolic signaling declines and whether detraining could influence it. It is crucial to understand that, initially, the study sought to compare two types of training: progressive load and constant load. However, a shortage of participants forced the researchers to pool the sample to investigate anabolic signaling proteins after an acute training session. Therefore, the analysis of the study design will focus on a single sample.

Purpose & Hypothesis
The study investigated the timing of the decline in anabolic signaling proteins (pmTOR S2448, pp70S6k T421/S424, and prpS6S235/236) in skeletal muscle. It also examined whether 10 days of physical inactivity could reverse this decline through increased phosphorylation of these proteins. Finally, it was examined whether rpS6 phosphorylation is regulated differently between type I and type II muscle fibers, and even within each type.
The researchers hypothesized that a multi-week training program would markedly decrease m-TOR signaling proteins, but that progressive overload would counteract this reduction in anabolic signaling. Furthermore, they expected to observe these changes at the level of individual myofibers and that a 10-day unloading period would significantly restore the altered signaling to baseline levels after the first training session.
What Did They Test and How?
Participants
The study concluded with a small sample of 14 healthy men, which was sufficient for the narrow research question. The researchers had estimated that they would be able to find significant differences with about 10 participants. However, the authors admitted that to analyze training load patterns, as mentioned above, they would have needed more than 42 participants.
Study Procedures
Training Intervention
For five weeks, participants completed 13 resistance training sessions targeting the vastus lateralis muscle. The program consisted of three sets of leg extensions and leg presses, with timed rest periods between sets and exercises. After a 10-day detraining period, muscle biopsies were taken at five specific sites.
Figure 1 Study Overview.
T0: 5-7 days before the first training session (baseline)
T1: 45 minutes after the first training session
T7: After the 7th training session
T13: After the 13th training session
X-T14: 10 days after final training session
Measurements
Muscle analysis: Samples of the vastus lateralis muscle were obtained by biopsy and analyzed for molecular changes using the following techniques.
Western blotting: This is a common microbiological technique for analyzing protein concentrations in muscle tissue. This study used Western blotting to measure concentrations of pmTOR S2448, pp70S6k T421/S424, and prpS6S235/236 in muscle tissue. Although variations exist, the general principle of the technique is as follows.
Immunohistochemistry: Immunohistochemistry was performed on frozen cross-sections of muscle tissue to specifically identify prpS 6 235/236 fibers.
Quantification of sarcoplasmic prpS6S235/236 staining: Sarcoplasmic p-rpS6S235/236 staining was quantified in muscle tissue cross-sections. Eight to 12 digital images per time point and participant were analyzed, obtained with a 10x optical microscope. Measurement of each myofiber was performed using optical densitometry software.
Myofibrillar diameter: This was measured in five to seven digital photographs of each cross-section, captured at 20x magnification using a microscope and digital camera. Software was used to delineate the inner borders of selected myofibers. Due to the absence of type IIX fibers in some participants and at some time points, these were excluded from the myofibrillar diameter analysis.

What Did They Find?
Western Blotting Analysis
A significant increase in prpS6S235/236 was observed immediately after the first training session (ES: 0.521; p < 0.001) compared to baseline. This increase moderated after seven sessions and until session thirteen. Interestingly, after the detraining period, prpS6S235/236 levels increased again, being similar to those observed after the first session, and significantly higher than those observed after sessions seven and thirteen. Figure 1 illustrates these results using box plots.
Figure 1 Box-Whisker Plots for prpS6S235/236 Protein Western blotting analysis
Data normalized to the total protein levels for each time point and individual participant. Normalization to the baseline (T0) was performed by dividing all time points of individual participants by the total average of T0. Differently colored points represent data from individual participants from each original training load group. p < 0.05; * p < 0.01; *** p < 0.001 with respect to T0; # p < 0.05 with respect to T1.
Phosphorylation of pp70S6k at the T421/S424 sites responded similarly to that of prpS6S235/236, with a modest effect (ES: 0.56). However, the increase following X-T14 treatment was less pronounced and showed less variability at pp70S6k T421/S424. The changes observed at these sites are illustrated in Figure 2.
Figure 2 Box-Whisker Plots for pp70S6k T421/S424 Protein Western blotting analysis
Data normalized to the total protein levels for each time point and individual participant. Normalization to the baseline (T0) was performed by dividing all time points of individual participants by the total average of T0. Differently colored points represent data from individual participants from each original training load group. p < 0.05; * p < 0.01; *** p < 0.001 with respect to T0; # p < 0.05 with respect to T1.
mTOR phosphorylation at S2448 showed an acute increase at time T1 compared to time T0. An upward trend in mTOR levels was observed throughout the study, as clearly illustrated in Figure 3.
Figure 3 Western blotting analysis of acute responses of phosphorylated and total levels of mTOR
Data normalized to the total protein levels for each time point and individual participant. Normalization to the baseline (T0) was performed by dividing all time points of individual participants by the total average of T0. Differently colored points represent data from individual participants from each original training load group. p < 0.05; * p < 0.01; *** p < 0.001 with respect to T0; # p < 0.05 with respect to T1.
Fiber-Type-Specific Analysis of prpS6S235/236
Figure 4 presents images of muscle cross-sections stained for prpS6S235/236, revealing a notable presence of this protein in type I, IIA, and some IIX muscle fibers. Fiber-type-specific analysis confirmed a similar regulation pattern between type I and II fibers, consistent with the results of Western blot analysis. Due to the demands of single-fiber analysis, data from T7 were excluded, focusing on the key time points of the study (T0, T1, T13, and X-T14). As seen in Figure 5, type I and II fibers did not show a significant decrease in prpS6S235/236 during training. However, detraining caused a significant increase in prpS6S235/236 in type II fibers compared to T13 (p < 0.05).
Figure 4 Muscle cross-sections from one participant stained for prpS6S235/236and the consecutive cross-section stained for type I, type IIA, and IIX stained myofibers
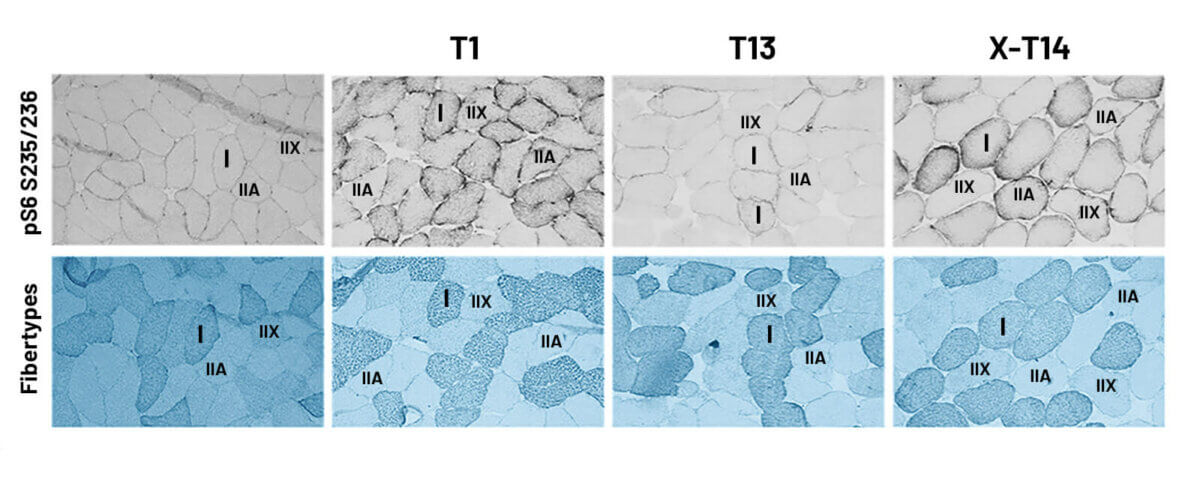
Figure 5 Box-whisker plot for all analyzed type I and II myofibers stained for prpS6S235/236
I Fiber-type-specific prpS6S235/236 data were normalized to the baseline (T0) by dividing all time points of individual subjects by the total average of T0. Differently colored points in the graphs represent data from individual subjects from the PR and CO groups. p < 0.05; * p < 0.01; *** p < 0.001 with respect to T0; # p < 0.05 with respect to T1; $ p < 0.05 with respect to X-T14.
Muscle Fiber Type Diameter Changes
Figure 6 shows a significant and similar increase in the diameter of both fiber types. Type I exhibited a slightly greater effect (ES: 0.34), and this growth was maintained after ten days of detraining.
Figure 6 Myofiber Hypertrophy via Analysis of Type I and Type II Fiber Diameter.
Bar graphs display the mean ± standard error of the mean. Normalization to the baseline (T0). Differently colored lines represent participants from the constant loading and progressive loading conditions in the original study design. *p < 0.05 with respect to T1.
What Do the Findings Mean?
This pioneering human study reveals the mechanism by which certain components of anabolic signaling become less responsive to resistance training but regain sensitivity after a period of rest. Specifically, seven training sessions (three times per week) markedly decreased rpS6 and p70S6k phosphorylation at 45 minutes post-exercise. After ten days without training, this phosphorylation increased, indicating a reactivation of the anabolic response. These results corroborate a robust study in rodents, which showed a similar reduction in rpS6 and p90S6k phosphorylation after 12 sessions of electrical stimulation, with recovery after 12 days of inactivity. Remarkably, detraining was sufficient to restore anabolic sensitivity without causing atrophy in muscle fiber diameter (Figure 6). The findings suggest that frequent rest could maximize muscle growth, but this is based on mechanistic studies with limited practical applicability. More research is needed to understand the implications for training recommendations. However, human studies indicate that detraining for up to three weeks does not significantly affect muscle mass, and that even reducing training volume to 1/9 can maintain it. One study showed that young people were able to maintain muscle mass with just 1/9 of their usual volume and increase it slightly with 1/3, suggesting that maintaining muscle requires less effort than building it.

It is important to highlight two key findings: the p70S6K/p70S6 pathway, an indicator of human muscle anabolism and dependent on mTOR, showed desensitization after a few weeks of training, unlike mTOR. This could be due to less mechanical tension on myofibers during detraining. Furthermore, the lack of direct measurement of MPS limits the interpretation of the results in terms of short-term muscle hypertrophy, as p70S6K/p70S6 desensitization does not necessarily imply lower MPS when mTOR is unaffected. Finally, given that "novice gains" typically extend over a longer period, this early desensitization of p70S6K/p70S6 is unlikely to significantly affect muscle growth during this period.
This cellular study reveals that muscle mass can persist during detraining. However, it remains unknown whether these periods are sufficient to restore cellular sensitivity to the level observed in novices. Research on the acute phosphorylation responses of mTOR-related signaling proteins during prolonged exercise, especially in humans, is limited and inconsistent. This area requires further attention. We appreciate the rigorous study design, which, despite having fewer participants than expected, contributed valuable and significant data to the field.

How Can You Apply These Findings?
Planned breaks and reducing training intensity are useful strategies for optimizing recovery and adapting to external factors that may influence progress. Maintaining muscle mass requires less effort than building it, suggesting that drastically decreasing training volume or even stopping it altogether may be beneficial for those looking to maximize muscle growth. Although more studies are needed to establish specific recommendations, it's important to remember that taking a week off for vacation or a period of high activity won't have a significant negative impact on your results. Even if you need to significantly reduce your training volume for an extended period, it will likely be enough to maintain the muscle mass you've already gained.
References
Jacko, D., Schaaf, K., Masur, L., Windoffer, H., Aussieker, T., Schiffer, T., Zacher, J., Bloch, W., & Gehlert, S. (2022). Repeated and Interrupted Resistance Exercise Induces the Desensitization and Re-Sensitization of mTOR-Related Signaling in Human Skeletal Muscle Fibers. International journal of molecular sciences, 23(10), 5431.
Norton, L. E., & Layman, D. K. (2006). Leucine regulates the translation initiation of protein synthesis in skeletal muscle after exercise. The Journal of nutrition, 136(2), 533S–537S.
Jefferson, L. S., & Kimball, S. R. (2001). Translational control of protein synthesis: implications for understanding changes in skeletal muscle mass. International journal of sport nutrition and exercise metabolism, 11 Suppl, S143–S149.
Yoon M. S. (2017). mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Frontiers in physiology, 8, 788.
Damas, F., Phillips, S., Vechin, F. C., & Ugrinowitsch, C. (2015). A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports medicine (Auckland, N.Z.), 45(6), 801–807.
Ogasawara, R., Kobayashi, K., Tsutaki, A., Lee, K., Abe, T., Fujita, S., Nakazato, K., & Ishii, N. (2013). mTOR signaling response to resistance exercise is altered by chronic resistance training and detraining in skeletal muscle. Journal of applied physiology (Bethesda, Md. : 1985), 114(7), 934–940.
Ogasawara, R., Yasuda, T., Sakamaki, M., Ozaki, H., & Abe, T. (2011). Effects of periodic and continued resistance training on muscle CSA and strength in previously untrained men. Clinical physiology and functional imaging, 31(5), 399–404.
Ogasawara, R., Yasuda, T., Ishii, N., & Abe, T. (2013). Comparison of muscle hypertrophy following 6-month of continuous and periodic strength training. European journal of applied physiology, 113(4), 975–985.
Bickel, C. S., Cross, J. M., & Bamman, M. M. (2011). Exercise dosing to retain resistance training adaptations in young and older adults. Medicine and science in sports and exercise, 43(7), 1177–1187.
Mitchell, C. J., Churchward-Venne, T. A., West, D. W., Burd, N. A., Breen, L., Baker, S. K., & Phillips, S. M. (2012). Resistance exercise load does not determine training-mediated hypertrophic gains in young men. Journal of applied physiology (Bethesda, Md. : 1985), 113(1), 71–77.
Terzis, G., Spengos, K., Mascher, H., Georgiadis, G., Manta, P., & Blomstrand, E. (2010). The degree of p70 S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. European journal of applied physiology, 110(4), 835–843.
Baar, K., & Esser, K. (1999). Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. The American journal of physiology, 276(1), C120–C127.
Comparte en redes sociales
Recent posts

A bad night's sleep: a reason to stay up even longer?

Creatine Effectiveness: What Does Science Say About Its Benefits?

Does meal timing help you lose fat?

Is your triceps press building muscle or holding you back?
Nutrition tailored to you: based on your genetic profile.

Carbohydrates: the key to an explosive workout.